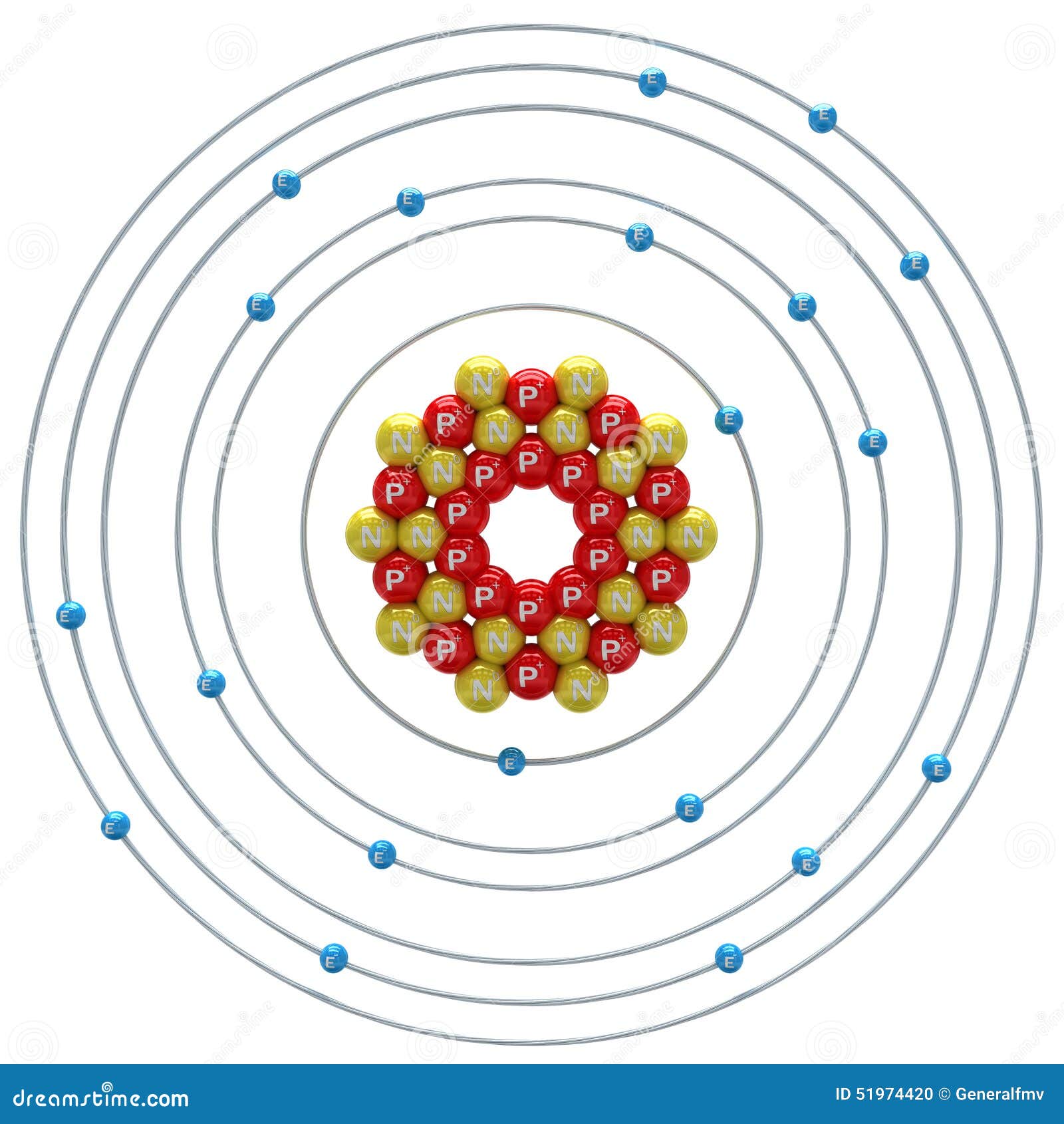
Calcium Atom on a White Background Stock Illustration Illustration of neutron, science 51974420
Since Bohr's model involved only a single electron, it could also be applied to the single electron ions He +, Li 2+, Be 3+, and so forth, which differ from hydrogen only in their nuclear charges, and so one-electron atoms and ions are collectively referred to as hydrogen-like atoms.The energy expression for hydrogen-like atoms is a generalization of the hydrogen atom energy, in which Z is.

How to Build a Model of a Calcium Atom Articles MerchantCircle
The Bohr model of calcium is a simplified representation of the atom's nuclear structure, named after Danish physicist Niels Bohr. It depicts the nucleus as a small, positively charged ball with electrons orbiting around it in circular paths at fixed distances.
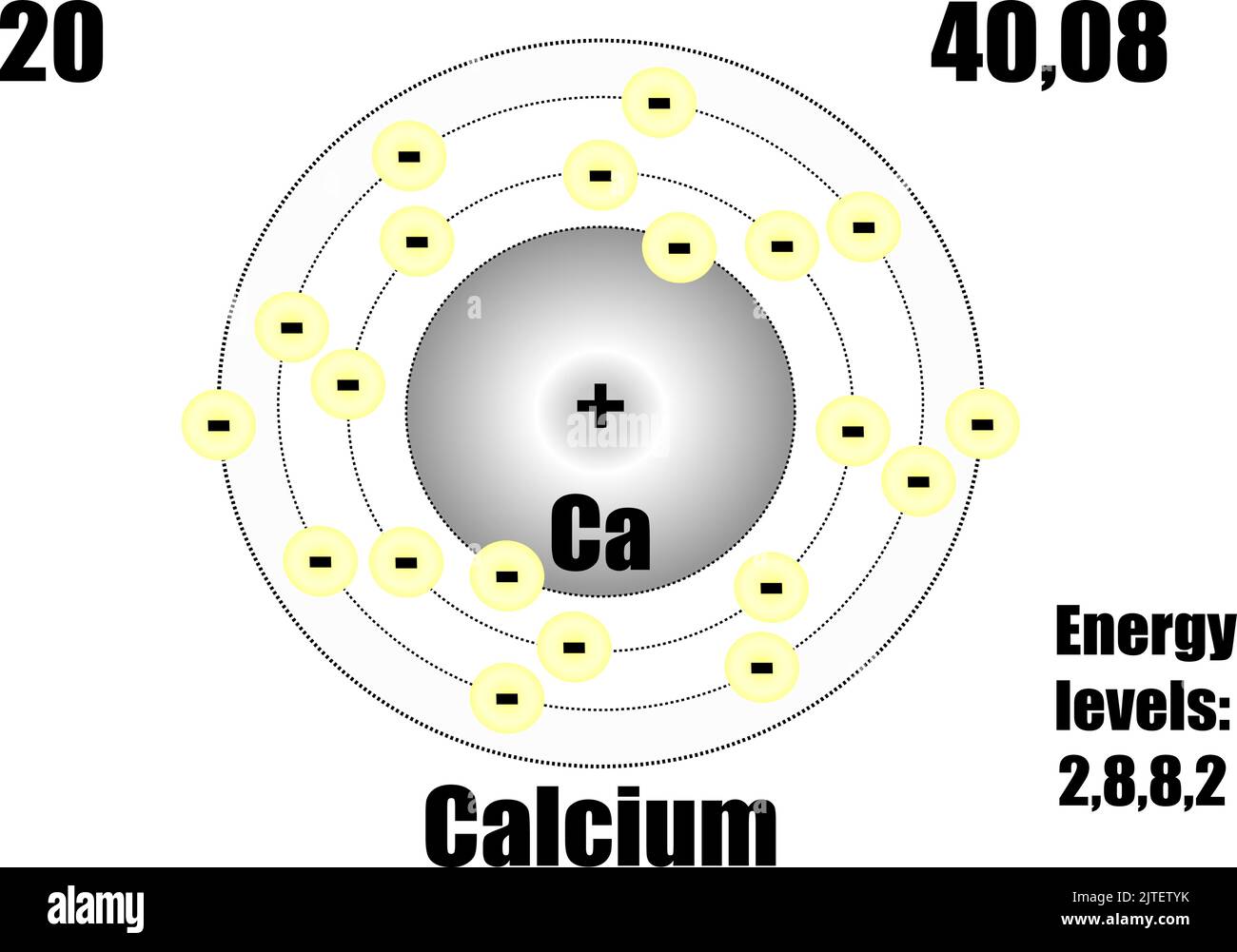
Calcium atom, with mass and energy levels. Vector illustration Stock Vector Image & Art Alamy
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.
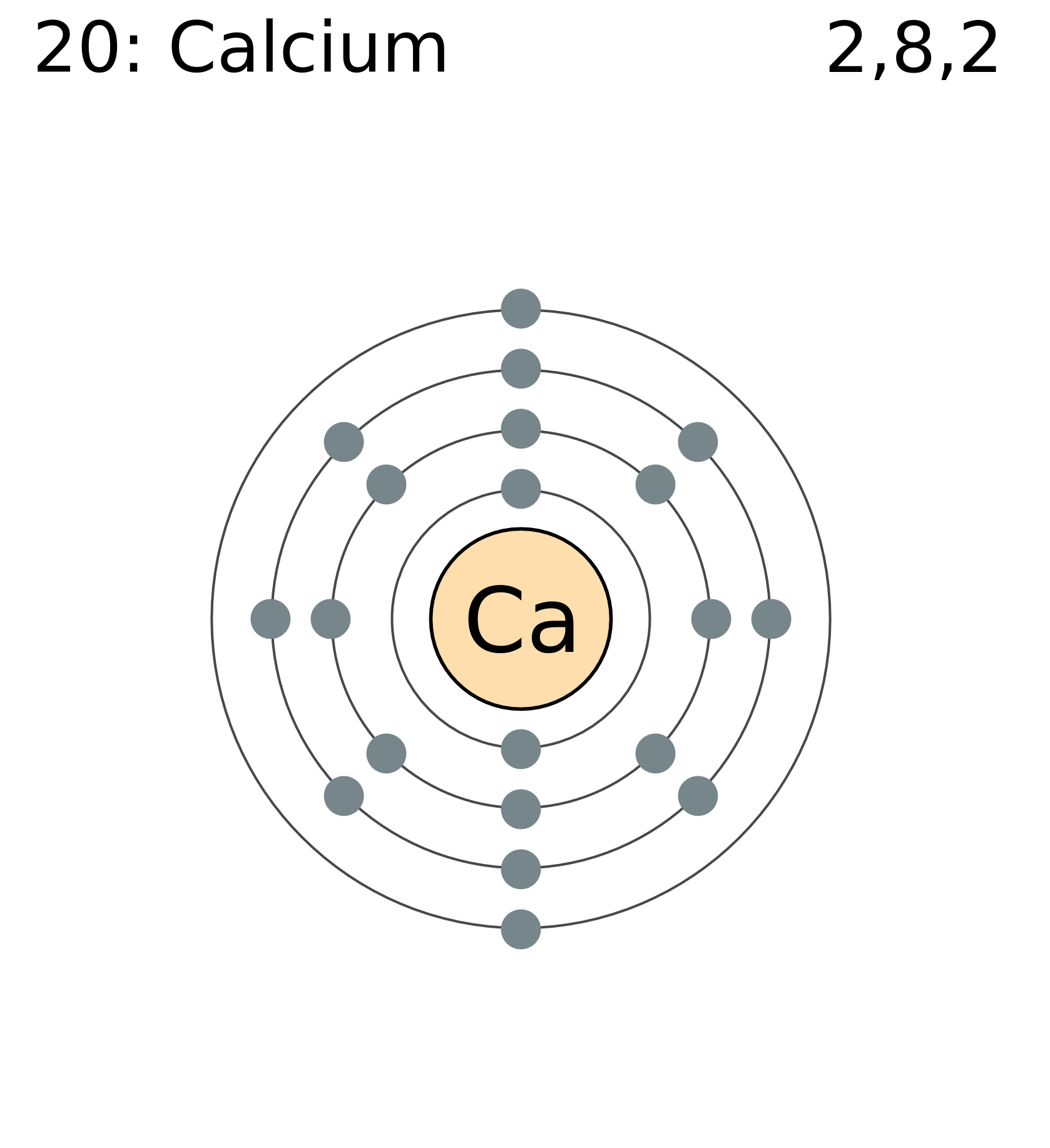
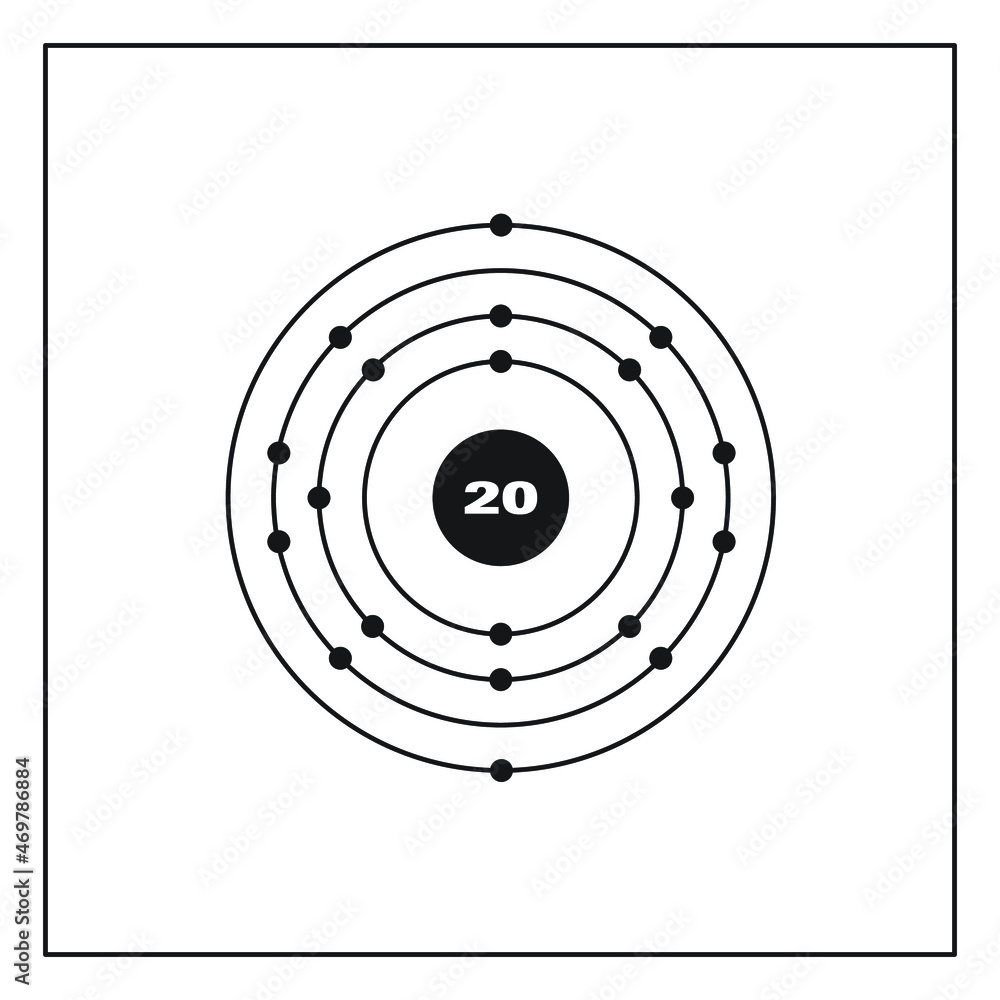
FileElectron shell 020 calcium.png
What is the Bohr model for calcium? A Visual Representation: A Bohr model is a way of visually representing what an atom of an element looks like. Atoms are tiny particles that make up.
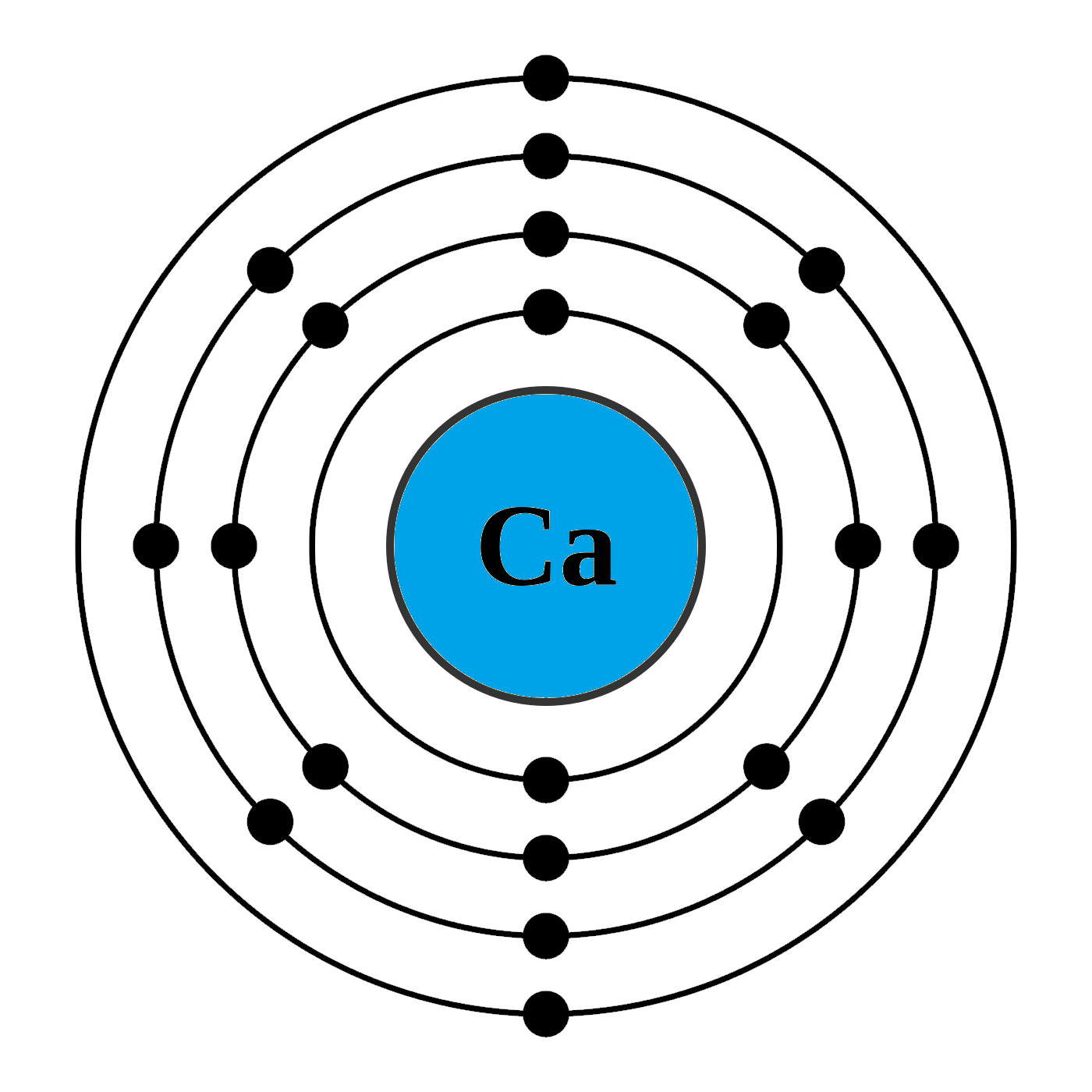
How can I draw electronic configuration of calcium in a shell nxwe70dd
One of the weaknesses of Bohr's model was that he could not offer a reason why only certain energy levels or orbits were allowed. Figure 10.4.1 10.4. 1: The energy levels of the electrons can be viewed as rungs on a ladder. Note that the spacing between rungs gets smaller at higher energies (CC BY-NC; Ümit Kaya)
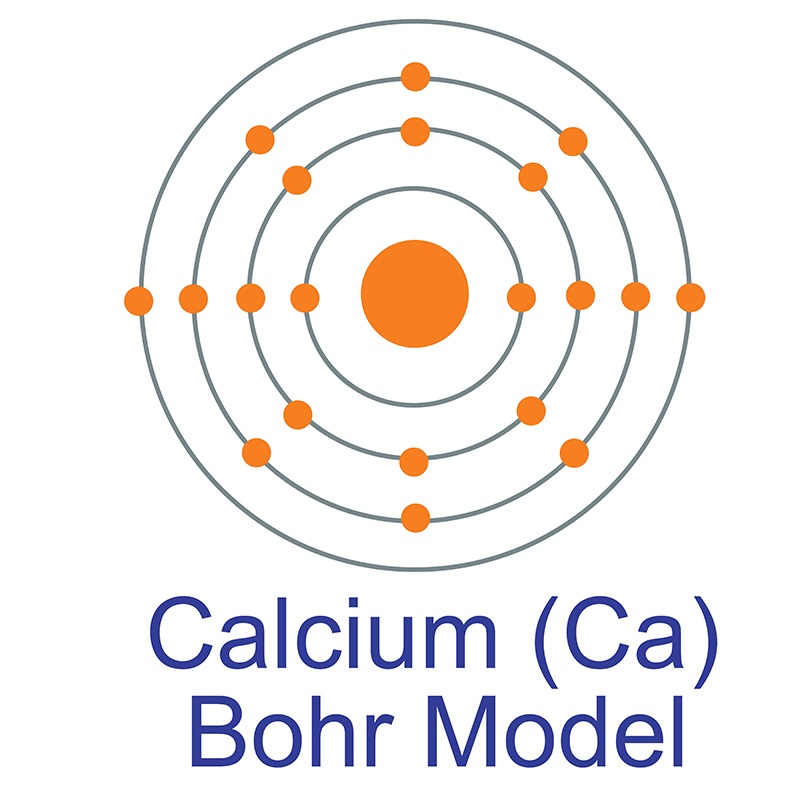
Calcium (Ca) AMERICAN ELEMENTS
In this video we'll look at the atomic structure and Bohr model for the Calcium atom (Ca). We'll use a Bohr diagram to visually represent where the electrons are around the nucleus.more.
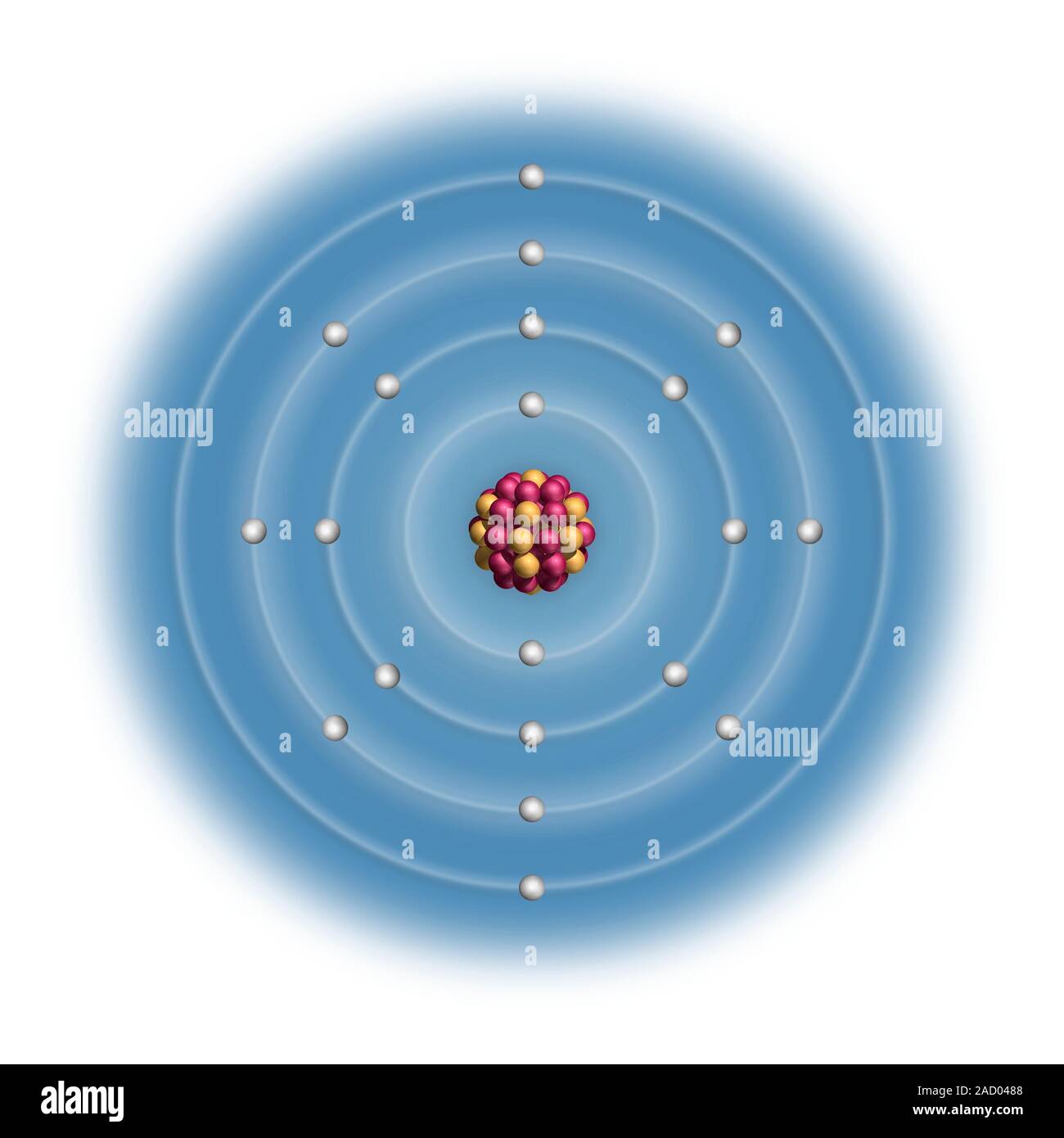
Calcium (Ca). Diagram of the nuclear composition and electron configuration of an atom of
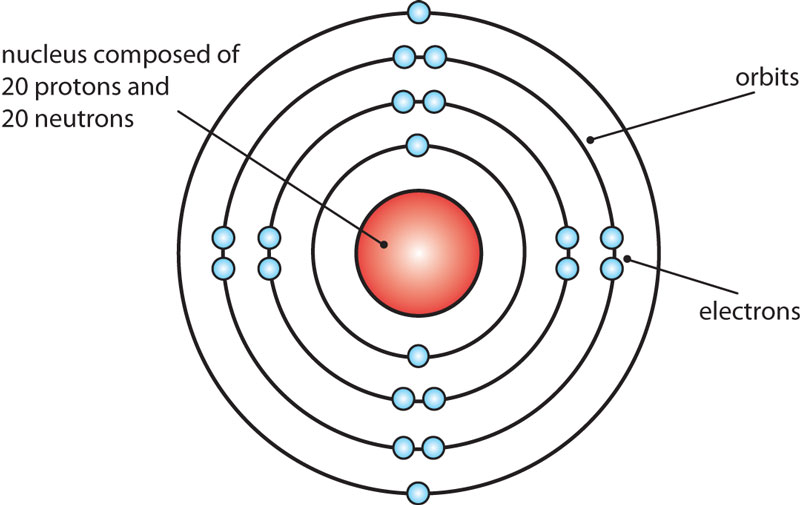
Basic Information Name: Calcium Symbol: Ca Atomic Number: 20 Atomic Mass: 40.078 amu Melting Point: 839.0 °C (1112.15 K, 1542.2 °F) Boiling Point: 1484.0 °C (1757.15 K, 2703.2 °F) Number of Protons/Electrons: 20 Number of Neutrons: 20 Classification: Alkaline Earth Crystal Structure: Cubic Density @ 293 K: 1.55 g/cm 3 Color: Silvery
Plakat Bohr model representation of the calcium atom, number 20 and symbol Ca. Conceptual vector
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model. Download this

182 Calcium Atomic Properties Images, Stock Photos & Vectors Shutterstock
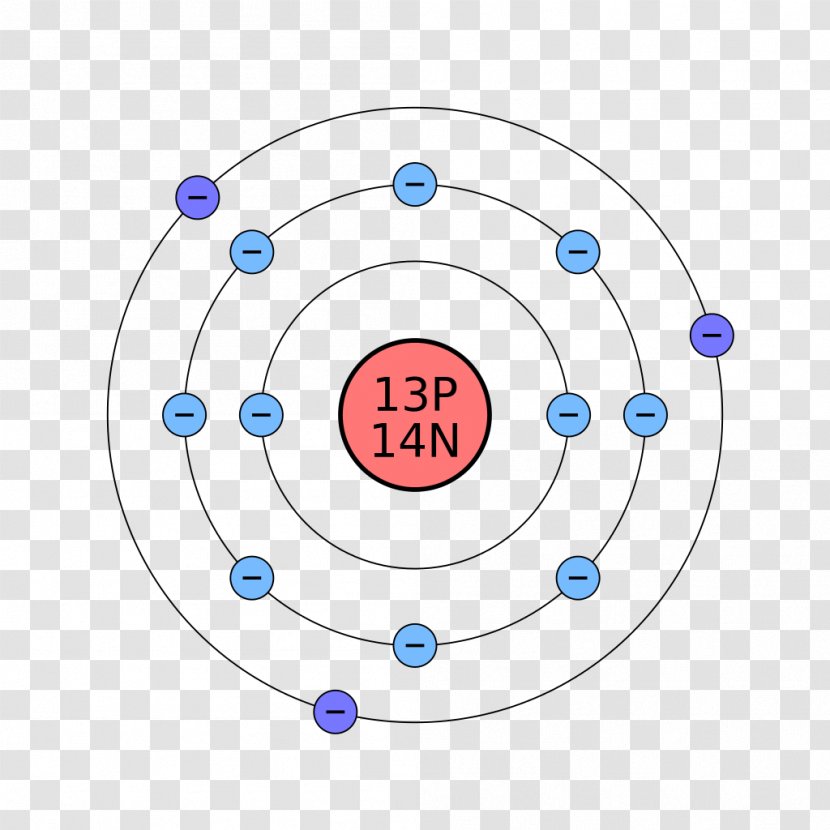
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com

Calcium Facts
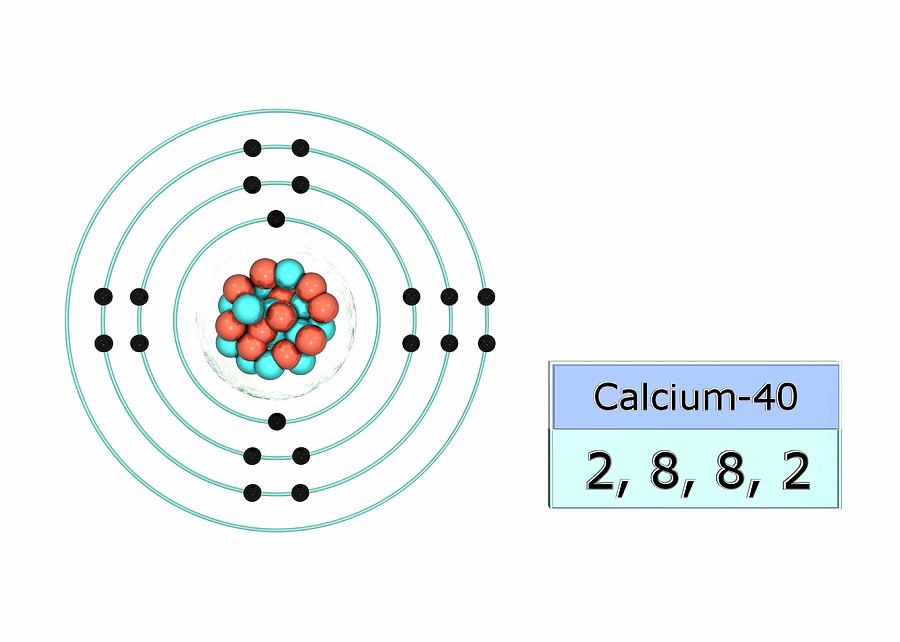
The Bohr Model of Calcium (Ca) has a nucleus that contains 20 neutrons and 20 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Calcium contains only 2 electrons that also called valence electrons. Page Contents show
Calcium Electron Dot Diagram Photos Cantik
Learn how to draw Bohr Models of atoms to further your fundamental understanding of Chemistry.
Bohr Model Atom Electron Configuration Argon Calcium Molecular Transparent PNG
Calcium (Ca) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Calcium atom electron configuration through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.

Calcium Stock Photo Download Image Now iStock
The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to atomic theory.

Calcium atom bohr model Royalty Free Vector Image
The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus.. Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of.
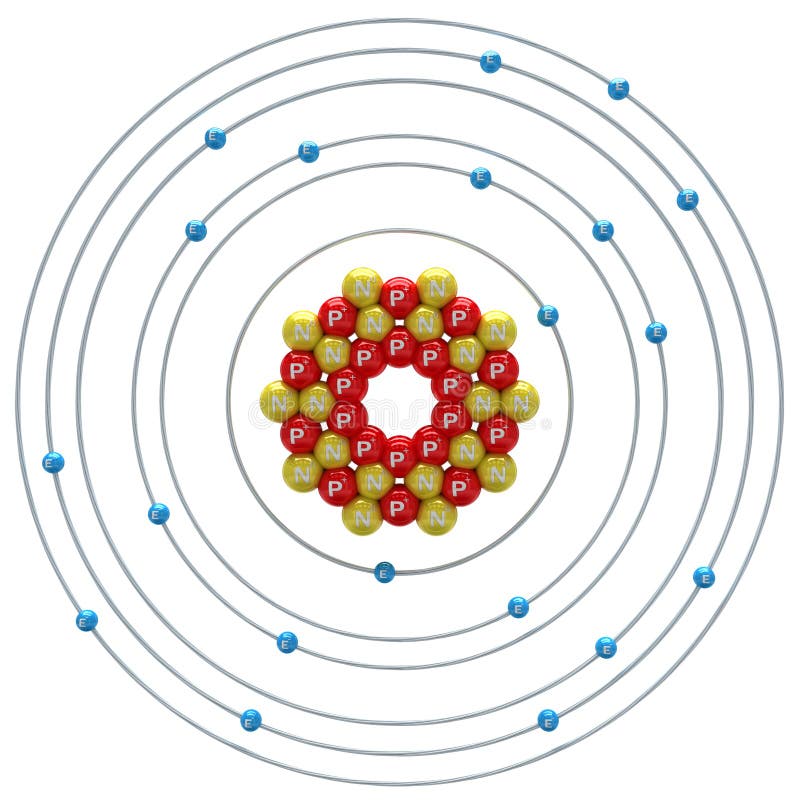
Calcium Atom on a White Background Stock Illustration Illustration of neutron, science 51974420
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
Calcium Bohr diagram Calcium
Course: Class 9 Chemistry (India) > Unit 4. Lesson 1: Models of an atom. Discovery of the electron and nucleus. Rutherford's gold foil experiment. Drawback of the Rutherford model. Bohr's model of an atom. Atomic structure. Science >. Class 9 Chemistry (India) >.